NeoPlex™ COVID-19 Detection Kit Assay
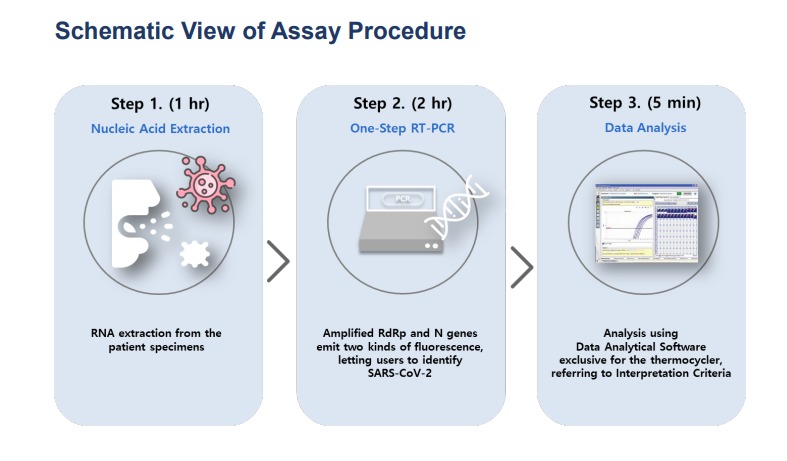
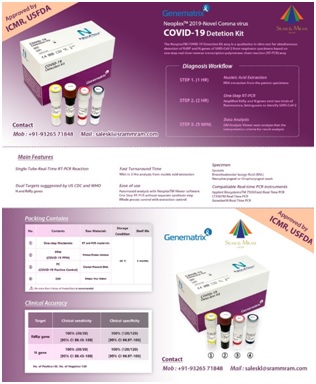
The NeoPlex™ COVID-19 Detection Kit Assay is a qualitative in vitro test for the simultaneous detection andconfirmation of RdRp and N genes in SARS-CoV-2 virus causing COVID-19 from upper respiratory specimens(suchas nasopharyngeal, oropharyngeal, mid-turbinate and nasal swab) and lower respiratory specimens (such assputum, BAL, and tracheal aspirate) from individuals suspected of COVID-19 by their healthcare provider. TheNeoPlex™ COVID-19 Detection Kit Assay is real-time reverse transcription polymerase chain reaction (rRT-PCR)assay. Testing requires a small sample volume and short hands-on time with results available in approximately 3hours. This test kit is intended for professional use.
• N gene
• RdRp gene
• Internal gene for whole process control Features
• Single-Tube Real-Time RT-PCR Reaction
• Dual Targets suggested by US CDC and WHO
• Fast Turn Around Time (3hrs)
• Ease of Use
• Compatible Real-Time PCR Instruments
• Applied BiosystemTM 7500 (Fast) Real-Time PCR
• CFX96TM Real-Time PCR
Results are for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 is generally detectable in respiratoryspecimens during the acute phase of infection. Positive results are indicative of presence of SARS-CoV-2 RNA;clinical correlation with patient history and other diagnostic information is necessary.
• Positive results do not rule outbacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
• Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patientmanagement decisions. Negative results must be combined with clinical observations, patient history, andepidemiological information.
The NeoPlexTM COVID-19 Detection Kit is intended for use by qualified, trained clinical laboratory personnelspecifically instructed and trained in the techniques of real-time RT-PCR and in vitro diagnostic procedures.
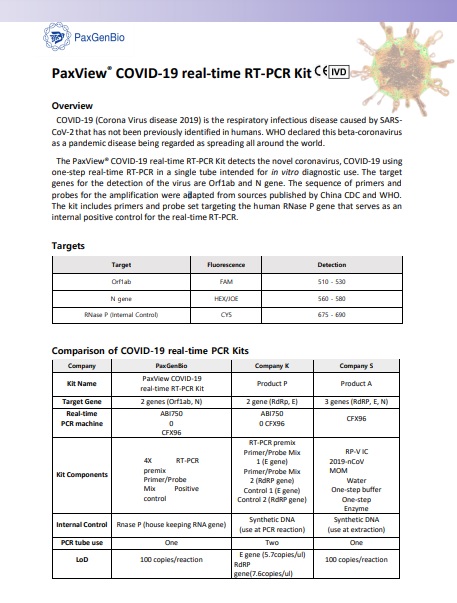
PaxView® COVID-19 real-time RT-PCR Kit
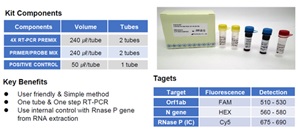
The PaxView® COVID-19 real-time RT-PCR Kit detects the novel coronavirus, COVID-19 using one-step real-time RT-PCR in a single tube intended for in vitro diagnostic use. The target genes for the detection of the virus are Orf1ab and N gene. The sequence of primers andprobes for the amplification were adapted from sources published by China CDC and WHO. The kit includes primers and probe set targeting the human RNase P gene that serves as an internal positive control for the real-time RT-PCR.
Sample Types:
1. Oropharyngeal Swab
2. Nasopharyngeal Swab
3. Sputum
4. Bronchoalveolar Lavage (BAL)
Reaction Time: 1.5 hours
Features:
1. User friendly
2. One tube & One step RT-PCR
3. User internal control with Rnase P gene from RNA extraction